ACCUTIP MICRONEEDLE ARRAY PATCHES.
One platform, a million opportunities.
Microneedle Array Patches (MAPs) have emerged as a highly promising solution for local parenteral drug delivery. MAPs leverage the skin’s unique properties, offering a minimally invasive, pain-free drug delivery technology with the potential to enhance therapeutic outcomes while improving patient comfort and compliance.
LTS proprietary Accutip MAP platform has evolved from years of investment in developing a Microneedle Array Patch technology from first principles to Phase 1 clinical trials.
The technology features microstructures (microneedles) to penetrate the outermost dermal layer to deliver a drug or vaccine to the top layers of the skin. The Accutip MAP platform is based on dissolvable microneedles and the active pharmaceutical ingredients (APIs) are incorporated therein. Configured specifically for the API and release option such as fast (within minutes or hours) or long-term (within days or months), each MAP can feature up to 600 needles per cm², with needle lengths ranging from 200 up to 1000μm.
At LTS we are focusing our technology on becoming the delivery route of choice for vaccines as well as for biologics, mRNA and small molecules. As a result, we have case studies demonstrating successful human clinical Phase I study with Hepatitis B Surface Antigen VLP MAP, a promising proof-of-principle pre-clinical study in guinea pigs with partner mRNA/LNP MAP and an equally exciting pre-clinical study in minipigs with recombinant Interferon-beta MAP.

Key benefits for patients
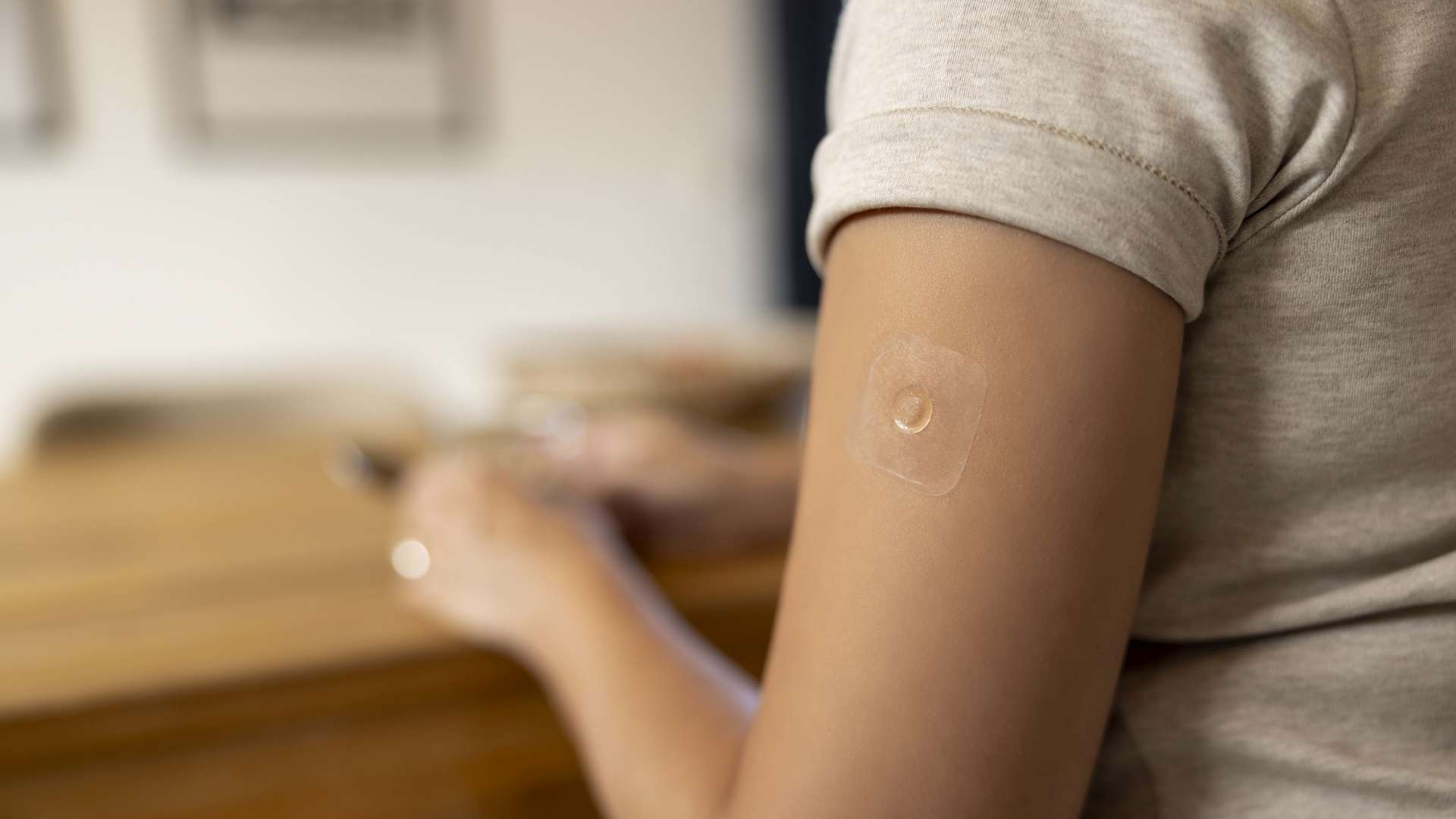
In addition to our CDMO offering, based on our proprietary Accutip MAP platform, we are also open to collaborations with organizations seeking a manufacturing partner based on other MAP technologies such as hydrogel-forming needles, coated microneedles, etc.