LTS receives grant of $1.2 Million to support the development of thermostable mRNA formulations for Microneedle Array Patches
Andernach – LTS, a leading pharmaceutical technology company, announced today that it has received a grant from the Bill & Melinda Gates Foundation. The grant will support new formulation methods for mRNA, such as dissolvable microneedle array patches (MAPs), to increase the thermostability of the overall product for its use in low- and middle-income countries (LMICs), especially in hard-to-reach conditions. This grant will fund $1.2 million over a duration of 15 months.
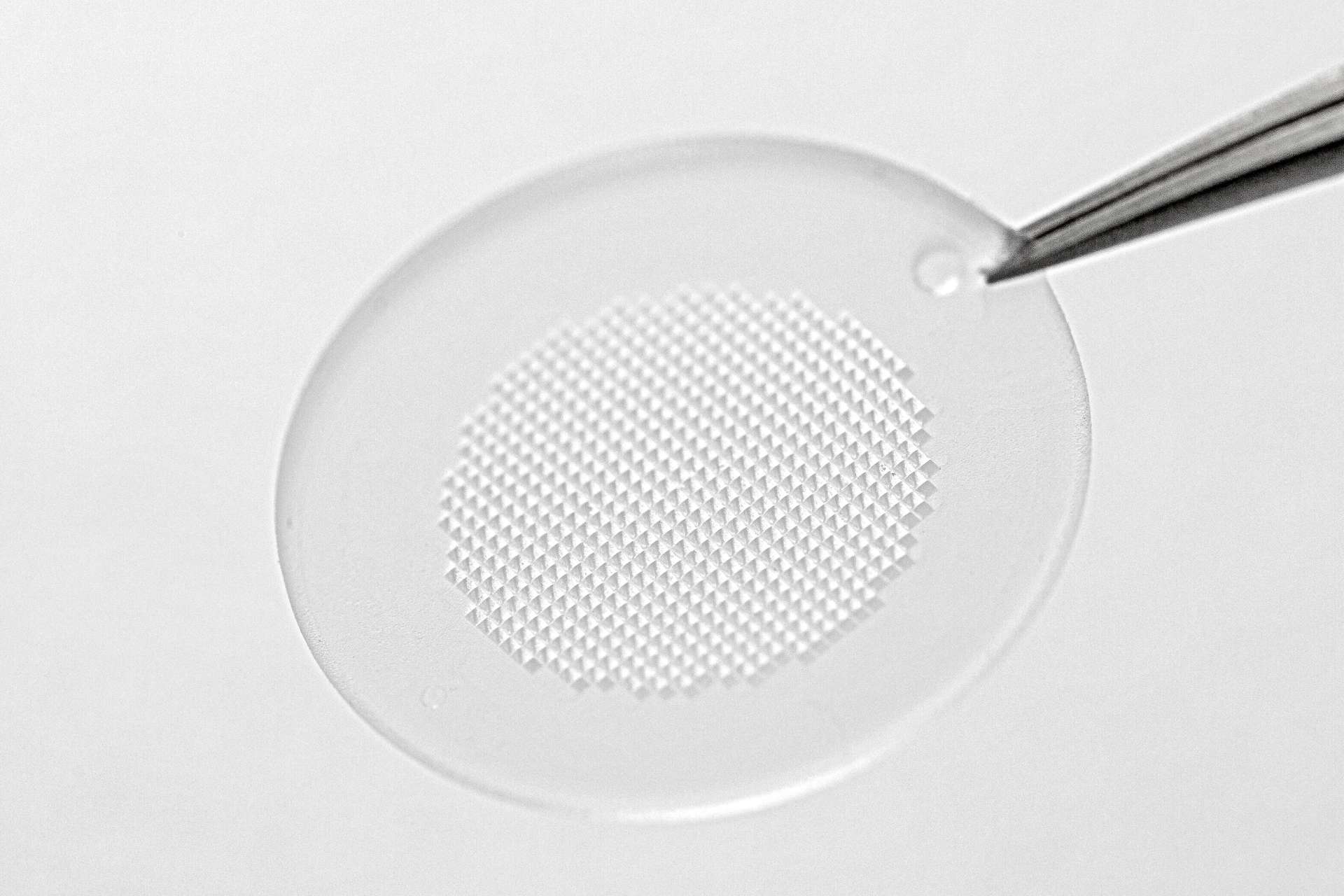
mRNA technology is considered a potential game-changer for a range of infectious diseases, including tuberculosis, malaria, and Lassa fever, which disproportionately affect people in LMICs. One of the major challenges the world faces in getting these life-saving vaccines to vulnerable populations – particularly those in poorer countries – is the need to store them at very low temperatures. The need for frozen storage of the current generation of mRNA vaccines is due to the fragility of mRNA molecules. The incorporation of mRNA into a dissolvable MAP may overcome this challenge by protecting the mRNA against degradation, removing the need for frozen storage, and simplifying the access to people living in LMICs.
At the World Vaccine Congress in Barcelona, LTS recently presented pre-clinical data from an mRNA/LNP MAP-based vaccination against rabies, from a collaboration with a biopharmaceutical company. This successful pre-clinical study with mRNA/LNP vaccine against rabies demonstrated that the cold-chain requirements can be reduced from -80ºC to 2-8ºC by using a MAP instead of an injectable formulation.
MAPs are an innovative drug delivery technology that offers advantages in comparison to established drug delivery applications, such as the opportunity for dose-sparing, lowering requirements for cold-chain and the possibility for self-administration.
Bas van Buijtenen, CEO of LTS, commented: “At LTS, we care passionately about bringing patient friendly drug delivery to people worldwide. We are honoured to be receiving support from the Gates Foundation in creating solutions for populations that would otherwise be at risk of being left behind. With MAPs, we aim to deliver improved access to essential vaccines to these groups, potentially saving lives.”
“The LTS MAP team is excited to have support from the Gates Foundation for the development of a thermostable mRNA/LNP MAP”, said Dr. Frank Theobald, Head of MAP Program at LTS. “LTS has made great progress recently with its MAP Program with respect to pre-clinical and clinical data, taking steps towards the up-scaling and commercialization of the MAP technology. LTS believes that MAPs will be a breakthrough technology for improving vaccination coverage in LMICs. Support from the foundation will help to develop the MAP technology further and bring it to the next level of maturity and make it accessible for patients in the foreseeable future.”
About LTS
We CARE. We CREATE. We DELIVER. The driving philosophy behind LTS. As a trusted technology partner for the pharmaceutical industry, we develop and manufacture innovative drug delivery systems such as Transdermal Patches (“TTS”) and Oral Thin Films (“OTF”) as well as wearable drug delivery devices (“OBDS”). LTS´ commercial offering encompasses more than 20 marketed products and a diverse pipeline of more than 40 development projects targeting multiple disease indications. LTS’s innovation pipeline contains both partner-funded as well as proprietary, LTS-funded projects. LTS maintains its leading position through the continuous refinement of its core TTS and OTF technologies and by advancing emerging drug delivery technologies, including Microneedle Array Patches (“MAP”) for the transdermal delivery of small and large molecules, biological actives and vaccines. With its SorrelTM wearable drug delivery platform LTS offers patient friendly solutions for complex drugs delivery at home. Founded in 1984, LTS operates today from four sites: in Andernach, Germany, West Caldwell, NJ, USA, St. Paul, MN, USA and Netanya, Israel. LTS has also a representative office in Shanghai, China.
Contact
Dr. Iris Schnitzler
iris.schnitzler@ltslohmann.com