LTS Advances Quality Oversight with Veeva Vault QMS
Leading pharmatech unifies quality processes for greater efficiency, collaboration, and compliance
LTS LOHMANN Therapie-Systeme AG (“LTS”), a leading pharmaceutical technology company, and Veeva Systems (NYSE: VEEV) today announced that they are partnering to advance LTS’ quality operations. LTS will use Veeva Vault QMS to improve process control and visibility, align global production sites, and increase audit readiness.
“As we continue to grow, we need a modern, scalable, and user-friendly quality management tool to harmonize and advance our quality processes across the organization,” said Dr. Heike Barnikow-Bock, corporate vice president operations, quality projects & consulting, LTS. “With Veeva Vault QMS, we will strengthen oversight and GxP compliance while aligning with the quality requirements of our customers.”
“Veeva’s open collaboration and flexibility to meet our needs make it the perfect partner,” said Christian Langer, corporate senior vice president of global quality at LTS. “The team’s breadth of experience and focus on execution made completing this project in such a short amount of time possible.”
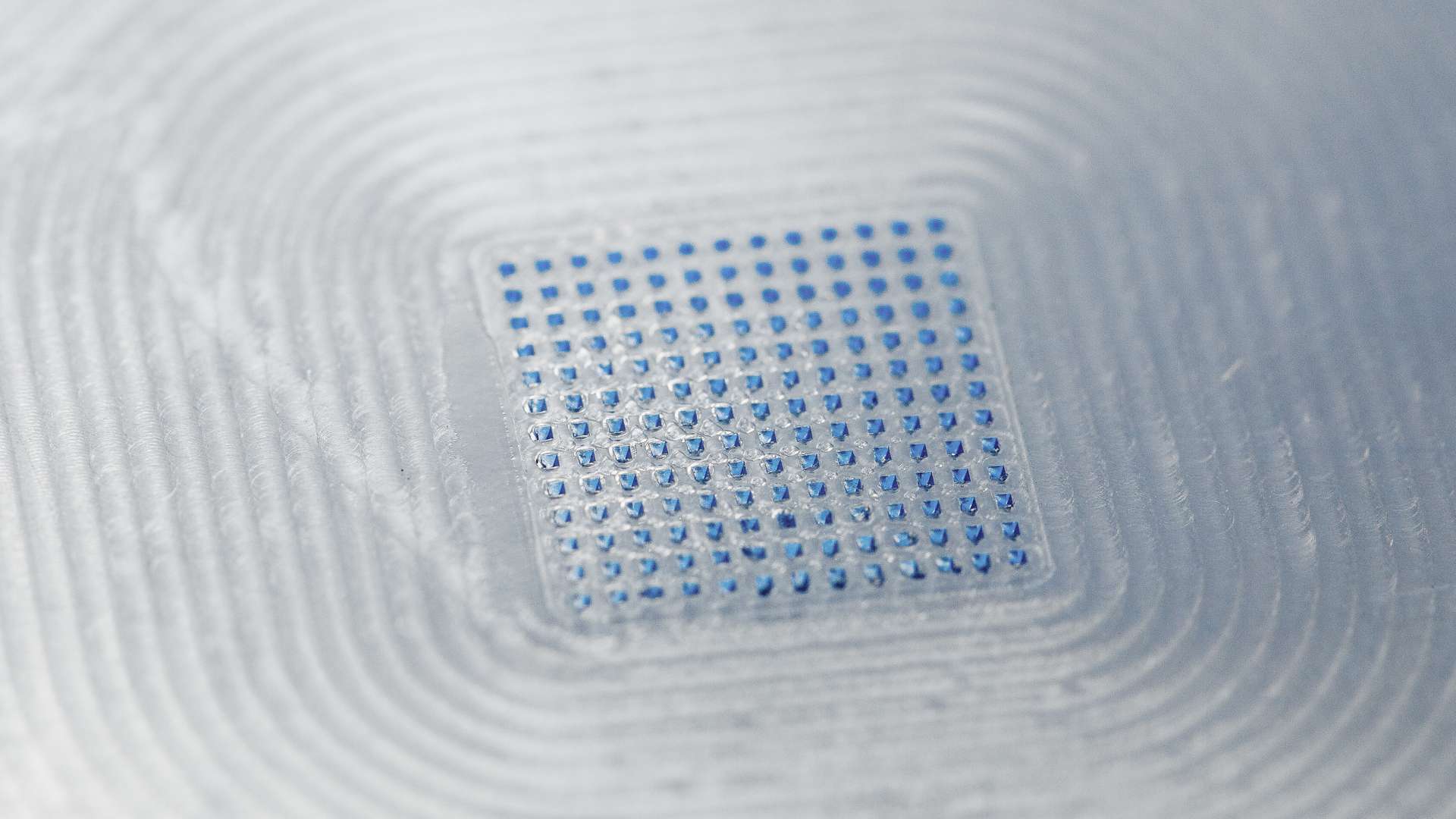
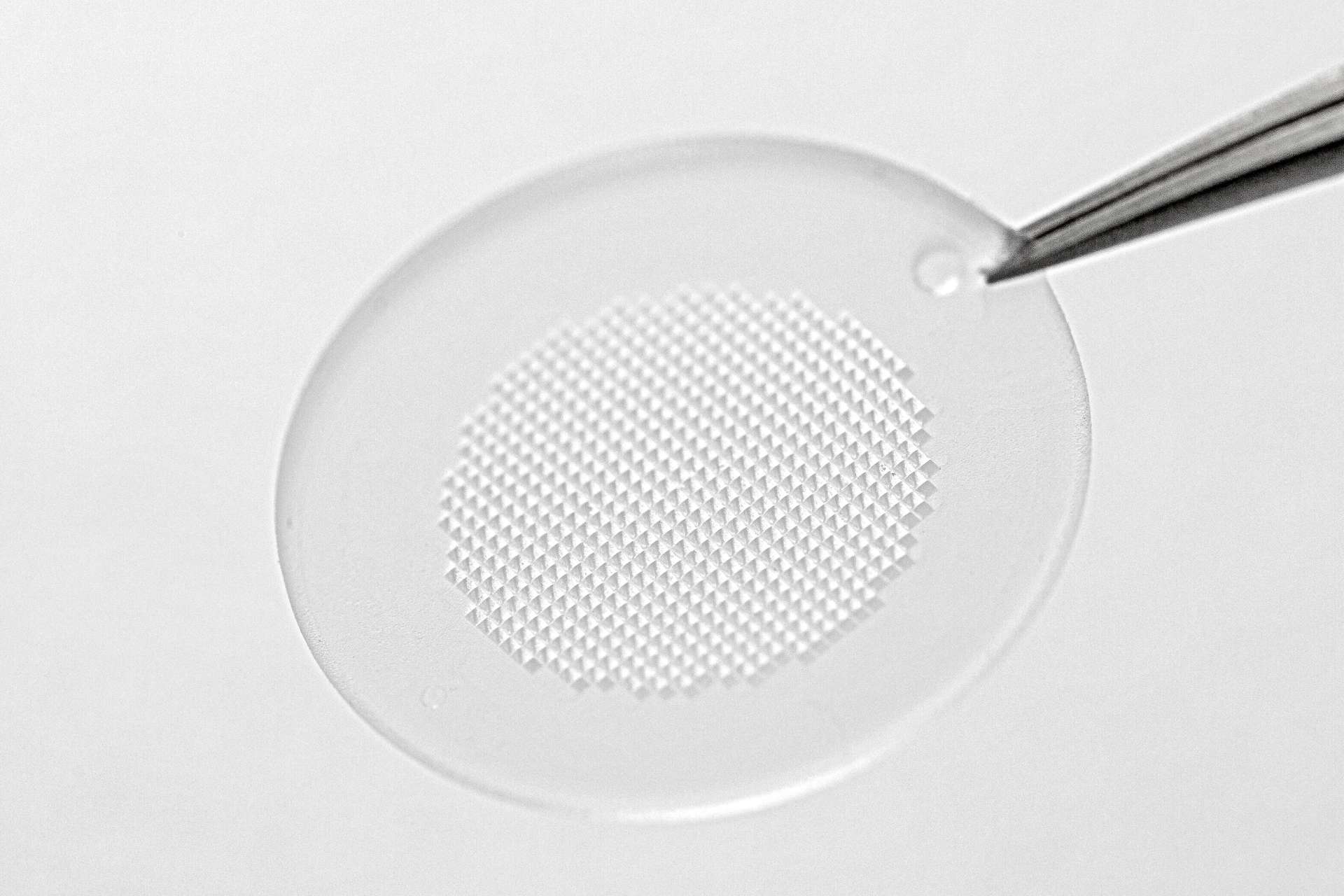
With headquarters in Germany and four sites in North America, Europe, and Asia, LTS develops and manufactures drug delivery systems, such as transdermal patches, oral thin films, micro array patches and wearable drug delivery devices. Vault QMS will help LTS manage and track quality processes, such as deviations, complaints, lab incidents, corrective and preventive actions, and change control. By improving its quality management processes, LTS can provide greater value to its partners and drive better patient outcomes, which aligns with the LTS purpose: “We CARE. We CREATE. We DELIVER.”
“Veeva Vault QMS helps companies like LTS simplify quality processes for better efficiency while ensuring ongoing inspection readiness,” said Patrik Brehm, director of quality strategy for Veeva MedTech in Europe. “Veeva is proud to partner with the team to help streamline its quality operations.”
Veeva MedTech offers unified, connected cloud software solutions to help deliver medical devices and diagnostics to patients in need. It includes Veeva Vault products across research and development and commercial to help speed the total product development lifecycle for medtech companies.
Veeva Vault QMS is part of Vault Quality Suite, which brings together quality systems and processes on a single cloud platform to enable efficient quality management.
About Veeva Systems
Veeva is the global leader in cloud software for the life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 1,000 customers, ranging from the world’s largest biopharmaceutical companies to emerging biotechs. As a Public Benefit Corporation, Veeva is committed to balancing the interests of all stakeholders, including customers, employees, shareholders, and the industries it serves. For more information, visit veeva.com/eu.
Veeva Forward-looking Statements
This release contains forward-looking statements regarding Veeva’s products and services and the expected results or benefits from use of our products and services. These statements are based on our current expectations. Actual results could differ materially from those provided in this release and we have no obligation to update such statements. There are numerous risks that have the potential to negatively impact our results, including the risks and uncertainties disclosed in our filing on Form 10-Q for the period ended July 31, 2023, which you can find here (a summary of risks which may impact our business can be found on pages 38 and 39), and in our subsequent SEC filings, which you can access at sec.gov.
About LTS
We CARE. We CREATE. We DELIVER. The driving philosophy behind LTS LOHMANN Therapie-Systeme AG. As a trusted technology partner for the pharmaceutical industry, we develop and manufacture innovative drug delivery systems such as Transdermal Patches (“TTS”) and Oral Thin Films (“OTF”) as well as wearable drug delivery devices (“OBDS”). LTS´ commercial offering encompasses more than 20 marketed products and a diverse pipeline of more than 40 development projects targeting multiple disease indications. LTS’s innovation pipeline contains both partner-funded as well as proprietary, LTS-funded projects. LTS maintains its leading position through the continuous refinement of its core TTS and OTF technologies and by advancing emerging drug delivery technologies, including Micro Array Patches (“MAP”) for the transdermal delivery of large molecule, biological actives. With its SorrelTM wearable drug delivery platform LTS offers patient friendly solutions for complex drugs delivery at home. Founded in 1984, LTS operates today from four sites: in Andernach, Germany, West Caldwell, NJ, USA, St. Paul, MN, USA and Netanya, Israel. LTS has also a representative office in Shanghai, China
Contact:
Dr. Iris Schnitzler
LTS LOHMANN Therapie-Systeme AG
Iris.Schnitzler@ltslohmann.com
Contact:
Jeremy Whittaker
Veeva Systems
+49-695-095-5486
jeremy.whittaker@veeva.com